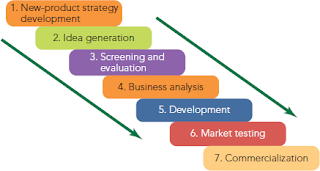
Top Challenges for Biopharmaceutical Process Development Industry in 2019

Biopharmaceutical is one of the most elegant and sophisticated achievements of modern science. The large, composite structures of these drugs look extraordinary in the 3-D modeling systems. Additionally, they also perform their tasks incredibly well, offering high value with fewer side effects. Today, various multinational pharmaceutical firms are slowly moving to biopharma on a large scale. However, with this comes several challenges. Considering the growth the industry has seen and the intricacies of biopharma, it is not surprising that major changes are needed to equip laboratories and operations in biopharma. Here are some of the major challenges that the biopharmaceutical Process Development industry is facing right now: Some of the leading nations that produce biopharmaceutical drugs lack research components and best manufacturing practices. Pharma firms must be built in a way that they are equipped with the best operational facilities. Cost pressure is expected t...